Surfactant Science
All surfactants have molecular structures that include both hydrophilic (water loving) and hydrophobic (water hating) parts. It is this feature which imparts surface activity to surfactants. When added to water, the hydrophilic parts are easily dissolved in the water whereas the hydrophobic parts are water insoluble. This results in the surfactant orienting itself at the interface between the water and the vessel holding the water and at the surface between the water and the air, with the hydrophilic part of the surfactant oriented towards the water and the hydrophobic part of the surfactant oriented away from the water.
Consider a drop of water on a freshly waxed surface. The freshly waxed surface is very hydrophobic, and water cannot easily wet the surface. The result is that the water associates with itself rather than the waxy surface, forming a bead on the waxy surface. If we were to add a little surfactant to the drop of water, the surfactant would migrate to the surface of the water drop, where the hydrophobic part of the surfactant molecule can easily associate with the waxy surface. Thus lowering the surface tension and allowing the water bead to spread out and wet more of the waxy surface.
This ability to reduce surface tension is a key measure of surfactant performance. The amount by which surface tension may be reduced is dependent upon several factors including the amount of surfactant which can exist at the water surface, referred to as the packing density. When a low level of surfactant is added to water some of the surfactant will dissolve in the water and most will migrate to the water surface. As more and more surfactant is added to the mixture, the solution and the surfaces both become saturated. When this happens additional added surfactant forms soluble aggregates with their hydrophilic parts oriented outward, toward the water, and their hydrophobic parts oriented inward, away from the water. These aggregates are called micelles. The concentration at which micelles start to form is called the critical micelle concentration or CMC. The surface tension for a given surfactant is minimized at or above the CMC. That is at or above the CMC no more surfactant can be accommodated at the surface and thus no further reduction in surface tension will occur.
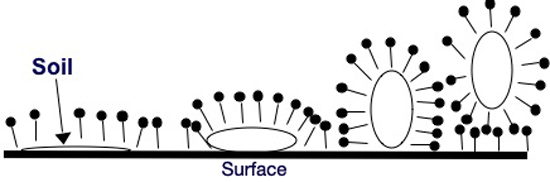
Imagine a surface that has an oily soil deposit. When this surface is added to a surfactant solution above the CMC, surfactant molecules will readily migrate to the fresh surface. The surfactant will also work itself between the oily soil and the surface effectively increasing the available surface area for surfactant. This process, which ultimately removes the oily soil deposit from the surface, is known as oily soil roll up. This is represented in the figure below. Individual surfactant molecules are represented by the pin shape in which the solid circle represents the hydrophilic head group and the straight line represents the hydrophobic tail group. The oily soil deposit is represented by the open circle.
Similarly, surfactants will surround or “wet” particulate soil. If the hydrophilic head group of the surfactant bears an electrical charge, then electrostatic repulsion between the similarly charged surfactant head groups of the surfactant layer on the surface and the surfactant layer surrounding the soil will help keep the soil suspended in solution and thereby help prevent redeposition of the soil on the surface.
Surfactants are commonly classified into groups based on the electrical charge on their hydrophilic head groups. Accordingly, surfactants are classified as anionic (the head group contains a negative charge), cationic (the head group contains a positive charge), nonionic (the head group does not contain a charge), and zwitterionic (the head group contains both a positive charge and a negative charge. The net charge on zwitterionic surfactants is pH dependant and accordingly their properties are also somewhat pH dependant.
Anionic surfactants account for the largest class of surfactants in terms of number of pounds of surfactant used. Widely used anionic surfactants include sulfonates, sulfates, ether sulfates and carboxylates. Anionic surfactants are the major surfactant in most hard surface cleaners, including manual dish wash products, all purpose cleaners, floor cleaners, bathroom tub and tile cleaners and a whole range of specialty hard surface cleaners. In general anionic surfactants are good foamers, although there are exceptions.
Nonionic surfactants include alcohol alkoxylates, alkylphenol ethoxylates, ethylene oxide propylene oxide block co-polymers, fatty acid amides, fatty acid esters and alkyl glucosides. In general, nonionic surfactants are good degreasers. Whereas some nonionic surfactants generate lots of very stable foam in use others are very good defoamers and thus used in applications such as automatic dishwashing where foam is a detriment to good cleaning.
Cationic surfactants tend to be very substantive on surfaces, especially those with longer hydrophobic tails. Accordingly, these surfactants are used as hair and fabric conditioners. Cationic surfactants with shorter hydrophobic tails often demonstrate biocidal properties and these cationic surfactants are the active ingredient in many disinfectant and sanitizer products.
In general, zwitterionic surfactants at moderate pH are relatively mild towards skin and as such these surfactants are commonly used in personal care products such as shampoos and body wash products. Zwitterionic surfactants, often called amphoteric surfactants, tend to form fairly stable foam and as such these surfactants are often included as a co-surfactant in products where a stable foam is desired. Commonly used zwitterionic surfactants include betaines, amphoacetates, amphopropionates and amine oxides.
While the nature of the polar head group is highly variable and determines many of the overall characteristics of the surfactant, it is the balance between the head group and the hydrophobic tail group that gives surfactants their surface activity. Hydrophobic tail groups fall into three broad categories, linear alkyl chains, aryl ring structures – often with linear alkyl chains attached, and oligimers of propylene oxide. The larger the hydrophobic group the less water soluble the surfactant and the higher the viscosity of the solution. The reduced water solubility leads to a lower critical micelle concentration and more efficient surface tension reduction.
Surfactant structures with short chain hydrophobes, for example xylene sulfonate and cumene sulfonate, have very little surfactant character. In spite of this, these materials are very useful as aids in solubilizing surfactants with relatively poor water solubility. Materials such as these are called hydrotropes.
For a more detailed discussion of surfactants, their structures and their properties we refer the interested reader to the Surfactant Science Series of text books published by Marcel Dekker Inc. This series of textbooks contains over 70 volumes discussing all aspects of surfactant science.